What is Diffusion?
Diffusion of gases and liquids is a very common phenomenon in nature. We see that the storms move, the cloud flies,sugar crystals dissolve in water forming solution, the fragrance of a flower spread to a distant place and so on. All these happen because of the movement of their component molecules from one place to the other, which is nothing but a process of diffusion.
When a solute is dissolved in a solvent it forms a solution. In a solvent the molecules or ions are having a random and spontaneous movement and when a solute is put into in the solute molecules or ions also move spontaneously. This random and spontaneous movement of ions throughout the medium is known as diffusion.Diffusion of molecules from a region of its higher concentration or pressure to a region of its lower concentration or pressure without the use of any external energy is a common tendency of any fluid substance.
Diffusion pressure
Diffusion pressure can be defined as a physical force of a substance by virtue of which its molecules or ions move from one place to another,if other conditions do not resist the movement.
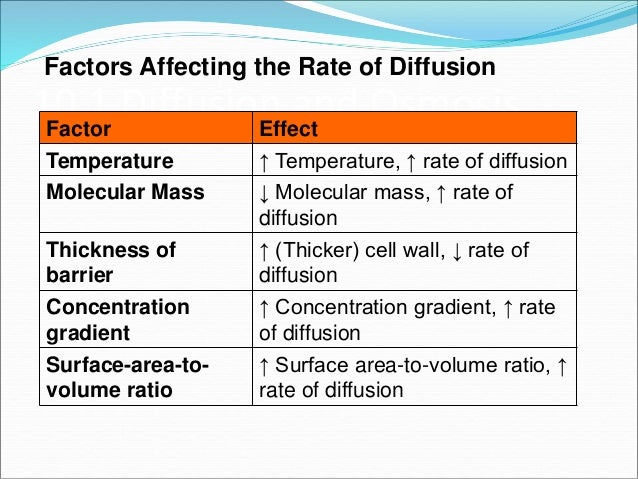
Factors affecting Diffusion
Diffusion or the rate of movement of any molecules is dependent on following factors.
- Temperature: Increase in the temperature increase the speed of the molecules, and thereby,the rate of diffusion too. In a similar way at lower temperature the rate of diffusion also decreases.
- Kinetic Energy of the Solvent Molecules: When the amount of surrounding medium or solution of a cell is increased the kinetic energy of a solvent molecules will be considerably more. As a result there will be more diffusion of solvent into the cell.
- Density: Density is defined as the amount of material that exists within a given volume. Regions of high density contain a greater number of particles per unit volume then regions of lower density. An increase number of particles leads to a greater chance of collisions, and this leads to an increased rate of diffusion. A lower number of particles leads to reduced chance of collisions and this lower the rate of diffusion. Therefore high density regions have a greater rate of diffusion than low density region.
- External Pressure: External pressure also determines the direction and rate of diffusion. If the external pressure on the surrounding medium of a cell increase the rate of diffusion of solvent molecules into the cell will also increase and the vice-versa.
- Size of the Molecules: With the increase of the size of the molecules the rate of diffusion decrease. The smaller the molecules the more is the rate of diffusion.


No comments:
Post a Comment